FEWER THAN 100 YEARS AGO : I
- polinaselin

- Mar 15, 2023
- 14 min read
GOLDEN TOAD

When did it become extinct?
No golden toads have been seen since May 1989.
Where did it live?
The golden toad was only known from an area of cloud forest above the city of Monteverde in Costa Rica.
(Golden Toad—The golden toad was restricted to the cloud forest above the city of Monteverde in Costa Rica. It was last seen in 1989.)
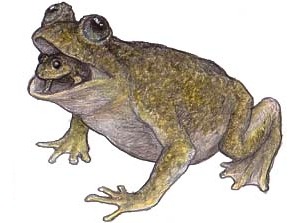
The disappearance of the golden toad was both mysterious and rapid. Only 25 years separate the species’ discovery by scientists in 1964 and the last sighting in 1989. Since its disappearance, this 5-cm-long toad has become an icon for the decline of amphibians the world over.
Unlike the majority of toad species, the male golden toad was brightly colored and shiny to the extent that it looked artificial. Th e species was also unusual as the male and female were very different in appearance. Th e male, with his magnificent golden orange skin, was in stark contrast to the larger female, who was black with scarlet blotches edged in yellow.
This toad was only known from a small area (around 10 km 2 ) of high-altitude cloud forest in Costa Rica that today is part of the Monteverde Cloud Forest Reserve. Th ese forests (also known as elfi n forests) are characterized by cloud, epiphytic plants galore, and small trees, which all in all give them a very primeval feel. In this small area of perpetually moist forest, the golden toad could apparently be encountered commonly and in large numbers, but only during the breeding season. Th e breeding season extended from April to June, when the rainy season is usually at its most intense. Th ese rains would fi ll the hollows around the bases of trees and other natural depressions with water—ideal toad breeding pools. Th e toads would collect around these pools in great numbers with the sole intention of breeding. Mating in any toad species is far from genteel, and golden toad breeding was a free for all. Th e males outnumbered the females by eight to one, and any female in the vicinity of a breeding pool soon found herself beneath a writhing mound of potential suitors. Th e males would get so excited and desperate that they would try to mate with anything that moved, including other males. Occasionally, between 4 and 10 feverish males would grab hold of each other to form a toad ball the significance of which is unknown—perhaps a female was in the middle of the ball but managed to give her suitors the slip. Once a male had struggled with his competitors and beaten them to get a good hold of a female in the breeding grasp known as amplexus, he could fertilize her eggs—or at least, this was his intention. Often, other males would come along and try to separate the mating couple so that they could get a chance at fertilizing the female’s eggs.

What with all this wrestling and bad sportsmanship, it’s quite surprising that the golden toad managed to breed at all, but breed they did, and the female would eventually lay 200–400 3-mm eggs in a long string in the breeding pool. Compared with many species of toad, the golden toad laid relatively few big, yolk-packed eggs, rather than lots of small ones, and it is thought this breeding strategy evolved because of the small size of the pools on which the toad depended. Th ese pools didn’t last very long, and so after the tadpole hatched, the race was on to change into a toadlet as quickly as possible. Th e abundant yolk in the eggs was the fuel for this rapid development.
After hatching, the tadpoles would spend around five weeks in the ephemeral pools before they lost their tadpole features and sprouted limbs, enabling them to begin their life on land. What the toads did outside of the breeding season is unknown. We don’t know what food they ate and how they went about catching it. Th e adults of the majority of other toad species are pretty unfussy when it comes to food, and they go for just about any creature that will fi t inside their capacious mouth. Th ere is no reason to believe the golden toad was any
different, but its small size restricted it to small animals like insects and other invertebrates.
Like much of the golden toad’s biology, we also have a poor understanding of why it disappeared. We know that when it was first discovered by Western scientists in 1964, it was found in large numbers, but in a very small area. In 1987, 1,500 adults were seen, but then in both 1988 and 1989, only one adult was seen. What happened to cause such a massive population crash? We don’t know for sure, but there are three main theories. It has been suggested that as the toad had such special breeding requirements—short-lived pools and a narrow window of opportunity—one erratic year of weather conditions would have completely scuppered their chance of a successful breeding season. Species like the golden toad have very specific habitat requirements, occupying very small ranges. Th is predisposes them to extinction as one little change in their environment can leave them with nowhere to go. Other scientists have suggested increasing amounts of ultraviolet (UV) radiation penetrating the atmosphere could have harmed the toads, but as they lived in dense forest shrouded in cloud during the breeding season, this is unlikely to be the cause of their demise. Th e last theory concerns the spread of chytrid fungi, which appear to make short work of amphibian populations wherever they become established. Drier conditions could have forced the toads into fewer and fewer ponds, increasing the transmission of this disease. With this said, it is possible that the golden toad still clings to existence in some remote corner of Central America.
• The cloud forests of Monteverde have lost 40 percent of their frog and toad species, and it is not only here that amphibians are in trouble. In the past three decades, scientists all over the world have reported massive declines in amphibian populations, with some 120 species thought to have become extinct since the 1980s. Th e declines and the extinctions are global, but the United States, Central America, South America, eastern Australia, and Fiji have been worst hit.
• Chytrids, a group of pathogenic fungi, are often blamed for this decline. Th is disease was
first noted on a captive frog in Germany, but its global spread has been linked to the trade in the African clawed frog, an animal that is used in laboratories the world over for a plethora of experiments. American bullfrogs have also spread around the world thanks to the pet trade, and these, too, carry the chytrid fungi, although they are not affected by the disease.
• Although the chytrids do cause disease and death in amphibians, it is unlikely they are wholly responsible for the global decline of these animals. Th ere are probably numerous factors at play, including habitat destruction, climate change, and increasing levels of UV radiation. Only intensive research will allow us to solve the puzzle and halt the decline of these interesting animals.
GASTRIC-BROODING FROG (Gastric-Brooding Frog—The first and only picture of a gastric-brooding frog “giving birth.)

When did it become extinct?
This frog was last seen alive in 1981.
Where did it live?
Th e gastric-brooding frog was known only from the Canondale and Blackall mountain ranges in southeast Queensland, Australia.
Another victim of the amphibian disaster was a fascinating little frog from Australia that was only discovered in 1973, yet by 1981, it had vanished without a trace. Th e gastric-brooding frog was a small species; females were around 5 cm long, while males were smaller, at approximately 4 cm. It lived in forest streams and rocky pools, and for much of the time, it would hide beneath rocks on the bed of these water bodies, but when it left these rocky refuges and moved out into the fast-flowing water, it showed itself to be a very accomplished swimmer. Its powerful hind-limbs terminated in feet that were almost completely webbed, and these were used with good effect to propel the frog through the water. Th e big, protruding eyes of this frog were positioned well on top of its head, and this allowed it to survey what was going on in the air and on land, while its body was out of sight beneath the water. Although it was very well adapted to an aquatic existence, the gastricbrooding frog would often leave the water to hunt or to seek out a new stretch of stream.
Its favored prey were small invertebrates, such as insects, but unlike many types of frog, the gastric brooder did not have a long, sticky tongue to secure its prey; instead, it waited until its food was within range and simply lunged at it with an open mouth. With its prey partially trapped, the frog would shove the rest of the victim’s body into its mouth using its forelimbs. Even though this frog was a capable predator, it was very small, and it was a tasty morsel for a range of predators. Herons and eels were partial to this amphibian, but it did have a useful defense if it was grabbed by one of these animals: mucus. All amphibians have skin glands that produce mucus to keep their skin moist as well as for protection. Th e gastric brooder could produce lots of very slippery mucus, which made it very hard for a predator to get a good grip.
In most respects, the gastric-brooding frog was like most other frogs, but what set it apart was the way it reproduced. Mating was never observed in this species, but it is known that the female laid between 26 and 40 eggs and that these were then fertilized by the male. Again, this is the normal amphibian approach when it comes to breeding as fertilization in all these animals is external. It is not completely clear what happened next as it was never actually seen, but at some point after the eggs were fertilized, either when they were still eggs or when they had hatched into tiny tadpoles, the female swallowed as many of her off - spring as she could. To the uninitiated, this may have looked like maternal cannibalism, but in fact, this was part of this frog’s unique reproductive strategy. Th e eggs or small tadpoles slipped down their mother’s throat and ended up in her stomach, and this is where they grew. In all animals, the stomach is the organ that plays a major role in digestion. Cells in the lining of the stomach produce very strong acid that breaks down food into its component fats, proteins, and carbohydrates so that enzymes can begin their digestive work.

This harsh, acidic environment is hardly ideal for developing off spring, but over millions of years, these frogs evolved a couple of tricks that turned the stomach into a snug little capsule for their developing brood. It seems that the eggs and the tadpoles of this frog secreted a type of chemical known as a prostaglandin. Th is chemical blocked the cells of the stomach lining from secreting acid, and the walls of the stomach thinned. Th e young frogs turned the stomach into a cozy crèche. After six to seven weeks of developing in their mother’s alimentary canal, 6 to 25 tiny but fully developed froglets clambered out of their mother’s mouth to begin their own life in the big wide world. Th roughout this whole brooding period, with her stomach effectively shut down, the female frog was unable to feed, so after the departure of her young, her first consideration was probably finding some food.
In fewer than 10 years after its discovery, the gastric-brooding frog disappeared. Extensive searches of the mountain streams in the early 1980s failed to turn up a single specimen. When the species was fi rst discovered in 1973, it was considered to be quite common, but by 1981, not a single specimen was to be found—it was as though it had been spirited away. Like the golden toad of Costa Rica, exactly what happened to the gastric-brooding frog is unknown, but there have been several explanations, some of which are more plausible than others. Pollution of the mountain streams by logging companies and gold panners has been cited as a reason for the disappearance of this species, but tests on the stream water failed to show any significant pollution. Habitat destruction has also been mentioned, but the areas where this frog was found have been pretty well protected. With pollution and habitat destruction largely ruled out, we arrive at the specter of disease. Th e chytrid fungus has caused the deaths of amphibians all over the world. Th e fungus latches on to the body of an amphibian and takes root in its skin. Th e fungus forms cysts within the deeper layers of the skin and breaks down keratin, a protein in the cuticle of many vertebrates, including adult frogs and toads. Th e skin of an amphibian infected with this fungus begins to break down, and in severe cases, the disease can eat right into the deeper tissues. In these cases, digits, and even limbs, can be eaten away. Th is in itself is not fatal, but the ability of the skin to transport gases and prevent the entry of other harmful micro-organisms is probably impaired, and the victim dies a slow and probably very painful death.

• The species discussed here is actually the southern gastric-brooding frog. In 1984, a very similar species, the northern gastric-brooding frog ( Rheobatrachus vitellinus ), was discovered living in the Clarke Mountains near Mackay in central coastal Queensland. A year later, this species also suff ered a total population crash, and it has not been seen since. • Th e gastric-brooding frog was very vulnerable to extinction as its range was so small. It existed in one small corner of Australia and nowhere else on earth.
• The chytrid fungus is not native to Australia, but it has somehow been transported there either by the pet or laboratory animal trade. Th e gastric-brooding frog probably had little or no immunity to the chytrid fungi. In a situation like this, a disease-causing organism can spread very rapidly indeed. • In Darwin’s frog, the tadpoles develop in the vocal sacs of their father, a strategy that doesn’t involve periodic starvation like gastric brooding.
ESKIMO CURLEW

When did it become extinct?
The Eskimo curlew is thought to have become extinct around 1970.
Where did it live?
In the northern summer, the Eskimo curlew spent its time in the Canadian subarctic. Its wintering grounds were the Argentinean Pampas, south of Buenos Aires.
(Eskimo Curlew—In addition to existing in huge numbers, the Eskimo curlew annually tackled one of the most arduous migrations in the natural world.)
The story of the Eskimo curlew is a sad tale of greed and senseless waste and a perfect example of how destructive our species can be. Th e Eskimo curlew was a small wading bird, no more than 30 cm long, with an elegant, 5-cm-long beak. Like the other curlew species, the Eskimo curlew had a distinctive, beautiful call, and the Inuit name for this bird, pi-pi-pi-uk, is an imitation of the sound they made on the wing and on the ground.
The Eskimo curlew may have been a small bird, but it was one of the most accomplished globetrotters that has ever graced the skies. Like many other species of wading bird, this curlew spent its time between northern breeding grounds and southern wintering grounds. Traveling between the two was no mean feat, and the small birds had to embark on one of
the most complex and dangerous migrations in the animal kingdom. As the short, northern summer ended and the curlew’s young had been reared, the birds took to wing for the beginning of an arduous and dangerous journey. Its migration took it in an immense clockwise circle, starting from the subarctic Canadian tundra, through the Western Hemisphere and east through Labrador, down through the Atlantic and across the southern Caribbean. Th e birds continued this epic journey until they reached their wintering grounds on the Argentinean Pampas. Some of the migrating birds went even further, eventually reaching Chile. Th e birds would spend a few months in South America until the spring returned to the north and the pull of hundreds of thousands of years of habitual behavior forced them into the air, en masse, for the return leg. Th e return to the breeding grounds took them through Texas, Kansas, Missouri, Iowa, and Nebraska. Completing such an arduous migration, nonstop, was an impossible task, so the enormous flock often alighted to refuel. Th e prairies of the Midwest were favored refueling stops, and the birds used their long bills to probe the soil for insect eggs, larvae, and pupae. Interestingly, it is thought that these refueling stops were heavily dependent on the Rocky Mountain locust, another extinct animal that once lived in unparalleled aggregations.
The risks of this journey were varied and grave. Th e North Atlantic is ravaged by storms, and each year, many of the curlews were blown off course to fi nd themselves alone and hungry in the cold expanse of the North Atlantic. Some stragglers even found their way to Britain and the decks of Atlantic ships. It seems that the entire world population of Eskimo curlew lived and traveled as one immense fl ock, which, at its peak, probably numbered in the millions. Th ere is protection in numbers, but each year, many individuals were undoubtedly picked off by predators or perished due to exhaustion. Th ese risks were intensified massively when Europeans started to settle North America.

Because the curlew flew in such great flocks, the settlers called them prairie pigeons, recalling the enormous flocks of passenger pigeons that blotted out the sun in eastern North America. Th ere are accounts of an Eskimo curlew fl ock of 1860 measuring more than 1 km long and wide. Any animal that is edible and exists in huge numbers quickly attracts the attention of hunters, and unfortunately, the curlew was both of these things. Th e curlew may have seemed numerous, but the enormous fl ock the hunters preyed on was the entire global population of this bird, and hunting quickly took its toll. During the birds’ feeding stops on their long route north, the hunters would close in on the fl ock and, sensing danger, the birds would take to the wing, an eff ective defense against land predators and birds of prey but completely useless against shotguns. Th e birds were so tightly spaced as they left the ground that a single blast from a shotgun, with its wide spread of shot, could easily kill 15 to 20 individuals. Th e birds were shot in such huge numbers that countless numbers of them were simply left to rot in big piles. Th e rest were taken away, piled high on horse-pulled carts. Such senseless slaughter of the Eskimo curlew on its northbound journey was bad enough, but it was not long before the hunters turned their attention to the birds’ breeding grounds.
During the northern summer, in anticipation of their long migration south, the birds fed on the swarms of insects that plague the tundra in the fleeting warmth, and as a result, they grew very fat. Hunters called these well-fed birds “doughbirds,” and even these were not safe. Th e hunters would fi nd their roosting grounds and slaughter them under the cover of darkness, using lanterns to dazzle them and sticks to club them. Th e fattened birds that survived took to the wing for the start of their migration, but gales would often blow them into New England, and this was the signal for every man with a gun to come out and harvest the poor animals. In the 1830s and 1840s, the birds were blown off course and ended up in Nantucket. Th e populace killed the birds so mercilessly that the island’s supply of powder and shot ran dry, interrupting the slaughter.

Under such intense hunting pressure, the Eskimo curlew was doomed. In 1900, Paul Hoagland was hunting with his father near Clarks, Nebraska. Th ey scared 70 Eskimo curlews into taking fl ight and followed them to a newly plowed fi eld. Th ey killed 34 of the birds with four shots. In 1911, the same man came across eight of the birds, and he killed seven of them. Reduced from an enormous fl ock covering an area equivalent to around 38 football fi elds, this sorry collection of birds was the last to be seen in Nebraska. Since 1900, 20 Eskimo curlews have been collected by ornithologists, and in 1964, the last confi rmed individual of this species was shot in Barbados. Lonely individuals may still plow the old migration routes, but it is very likely this species is gone for good.
• Hunting undoubtedly had a huge eff ect on the Eskimo curlew, but it is also thought that agriculture played a role in its demise. Much of the fertile prairie, the curlew’s refueling ground, was turned over to agriculture, and many of the insects on which the birds fed dwindled in numbers. One example is the Rocky Mountain locust, which once lived in swarms of staggering dimensions.
• Birds that live in flocks depend on strength in numbers for protection. A lone curlew would stand little or no chance of evading predators during its arduous migratory flight. If any Eskimo curlews still remain, their continued survival will be fraught with danger and uncertainty.









Comments